Every now and then someone sends me an email that claims the difference between Coke and water, and how bad Coke is for you. To illustrate that, the email points out that Coke can be used to clean a toilet, help remove a rusty bolt, or remove blood from a highway accident.
One item, in particular, caught my eye:
The active ingredient in Coke is phosphoric acid. Its pH is 2.8. It will dissolve a nail in about 4 days.
This one entry screamed that it just can’t be true. Let’s examine it:
“The active ingredient in Coke is phosphoric acid.” — An active ingredient is defined as: “a component or mixture that performs the function of the product”. What exactly is the function of Coca-Cola? According to the Coca-Cola web site, Coca-Cola does not perform any function, other than to provide “true refreshment” (a bit vague of a function if you ask me). To that end, I would be inclined to believe that the sugar and water in Coke are more likely to be called “active ingredients” than phosphoric acid, although phosphoric acid does contribute to the taste of Coke.
“Its pH is 2.8.” — Its pH may very well be 2.8, but with the amount that is actually in a can of Coke, I’m sure that the other ingredients buffer it. Ever drink a glass of wine? Good quality wine has a pH of about 2.9. pH of 2.8 is about the pH of lemon juice. A pH of 2.8 might make your mouth pucker, but it won’t hurt you.
“It will dissolve a nail in about 4 days.” — What will? Based on elementary school English, the subject of the first sentence is phosphoric acid. The “it” in the second and third sentences are pronouns referring to the phosphoric acid in the first sentence. That means that phosphoric acid will dissolve a nail in about four days. What does that have to do with Coke? Is it the only ingredient in there? I have hydrochloric acid in my stomach with a pH of two…if I hold a nail in my hand for four days, will it dissolve?
So, now that we have dissected it, lets go ahead and test it!
Introduction
The purpose of this experiment is to test the validity of the “The active ingredient in Coke is phosphoric acid. Its pH is 2.8. It will dissolve a nail in about 4 days.” statement that is found in several e-mails making their way around the Internet. Specifically, will a nail left in Coca-Cola dissolve in about four days (96 hours).
The first thing that came to mind is that the e-mail did not specifically say which variety of Coke it was talking about. In the absence of a particular variety, I would assume that the plain old Classic Coca-Cola variety would be assumed. To be on the safe side, I included four of the most popular varieties of Coke: Classic Coke, Diet Coke, Vanilla Coke, and Cherry Coke. For good measure, I also included Pepsi, Dr Pepper, Sprite and some genuine, fresh, New York City tap water (it’s won taste tests!).
The second thing that came to mind is that the e-mail did not specifically say what kind of nail the nail is. It is steel? Brass? Lead? Keratin? I decided that the two most popular kinds (and the kinds most likely to be found in my apartment) were the standard steel, “wall mounting” nail, and the keratin based fingernail. I rummaged around my toolbox and found the former. The latter was a bit harder to come by. I had just cut my nails earlier in the week, and didn’t have much growth to make an effective experiment; however, my toenails were due for a trimming. Only problem with toenails is that when clipped, they have a tendency to fly across the room at a high rate of speed. This made gathering them up a bit of a challenge, but testing the veracity of the email claims are my contribution to society, and as such, a little discomfort would have to be endured.
The following table details the list of equipment used in this experiment. I’ve also included the ingredients listed for the sodas used in the experiment.
| Quantity | Item |
|---|---|
2 oz. |
Coca-Cola (Classic) |
2 oz. |
Diet Coke |
2 oz. |
Vanilla Coke |
2 oz. |
Cherry Coke |
2 oz. |
Pepsi |
2 oz. |
Dr Pepper |
2 oz. |
Sprite |
2 oz. |
NYC tap water, unfiltered, straight from the tap |
9 |
glass spice jars with tops |
9 |
steel, 1” nails |
9 |
toenails of various sizes from Josh |
1 |
8x Carl Zeiss loupe (for examining the results) |
1 |
Revlon toenail clipper (for clipping the toenails) |
3 |
Paper towels (for general spills, toenail clippings, etc.) |
1 |
small glass food preparation bowl |






December 14, 2003
Each spice jar was labeled with the name of the liquid that it was to hold. Two ounces of each liquid in the experiment was measured out and poured into the corresponding spice jar. A steel and keratin nail were randomly chosen and assigned to each jar. Each jar, steel nail, and keratin nail were photographed. The steel nail and keratin nail were examined under the 8x loupe and notes were made about their appearances.
At 9 p.m. the nails were put into the jars, and the tops were put on the jars. The tops were not screwed on tightly, but rather left a little loose to allow some gas to escape.
December 15, 2003
At 9 p.m., one day after starting the experiment, the nails in each jar were photographed, examined with the 8x loupe, and compared to the control samples and the notes made at the start of the experiment.
Both the steel nails and the keratin nails from the Coke, Diet Coke, Vanilla Coke, Cherry Coke, Pepsi, and Dr Pepper showed no signs of change, other than a darker color. The steel nail and keratin nail in the Sprite showed no sign of change other than a slightly duller color. The steel nail in the tap water showed some signs of surface rust, with the water near the nail a medium rust color. The keratin nail in the water showed no changes.
December 16, 2003
At 9 p.m., two days after starting the experiment, the nails in each jar were photographed, examined with the 8x loupe, and compared to the control samples and the notes made at the start of the experiment and after one day.
Both the steel nails and the keratin nails from the Coke, Diet Coke, Vanilla Coke, Pepsi and Dr Pepper showed no signs of change, other than the darker color seen yesterday. The steel nail and keratin nail in the Sprite showed no sign of change since yesterday. The steel nail in the tap water showed more signs of surface rust, about 50% of the outside covered with it. The water near the nail was a deep rust color, and there were these little rust colored specks all over the bottom of the jar. The keratin nail in the water showed no changes.
The steel nail in the Cherry Coke showed some signs of a slight corrosion on about one-eighth of the shaft of the nail. It was only evident on one side of the nail, and I’m not sure whether this was the side of the nail that was in contact with the glass jar or not.
December 17, 2003
At 10:30 p.m., three days after starting the experiment, the nails in each jar were photographed, examined with the 8x loupe, and compared to the control samples and the notes made at the start of the experiment and after one and two days.
Both the steel nails and the keratin nails from the Coke, Diet Coke, Vanilla Coke, Pepsi and Dr Pepper showed no signs of change, other than the darker color seen the past two days. The Diet Coke steel nail seems to be lighter than the other cola nails, but it is still darker than the control nail. The Sprite liquid has turned a slightly green/yellow color, and the keratin nail shows a slightly green/yellow tint. The steel nail in the Sprite looks dull, but otherwise, unchanged. The steel nail in the tap water showed more signs of surface rust, about seventy-five percent of the outside covered with it, but there is a section that has no rust, again, I assume this is where the nail is in contact with the glass. The water near the nail was a deep rust color, and there were these little rust colored specks all over the bottom of the jar. The keratin nail in the water sports a rust colored hue, but otherwise, appears unchanged.
The steel nail in the Cherry Coke does not show the signs of the slight corrosion mentioned yesterday, and looks very similar to the other cola nails.
December 18, 2003
At 11:30 p.m., four days after starting the experiment, the nails in each jar were photographed, examined with the 8x loupe, and compared to the control samples and the notes made at the start of the experiment and after one, two, and three days.
This is the official end of the experiment since the e-mail said the nail would dissolve in Coke after four days. It has been four days since the experiment began.
The keratin nails from all of the sodas showed no ill effects. They did turn the color of the soda and were soft, like after you take a shower, but overall, they were unaffected.
The steel nail results are as follows:
| Jar | Result |
|---|---|
| 1. Coke | It is dark and rough to the touch. |
| 2. Diet Coke | It is smooth and is rusted on seventy-five percent of the shaft. |
| 3. Vanilla Coke | It is smoother and slightly lighter in color than that of the Coke nail. |
| 4. Dr Pepper | It is smooth and has an even, dark luster throughout the nail. Of the cola nails, this one seems to be the most consistent in its feel over the whole nail. |
| 5. Pepsi | It has the same color and touch as the Coke nail. |
| 6. Sprite | It is dull compared to the control nail. Otherwise, unchanged. |
| 7. Cherry Coke | It is slightly darker than the Vanilla Coke nail, but not as dark as the Coke or Pepsi nail. Has a slightly rough touch to it. |
| 8. Water | It has rust over 100% of it. Some patches are darker than others. |
| 9. Control | Nail is shiny and has the same color and touch as a nail straight from the package. |

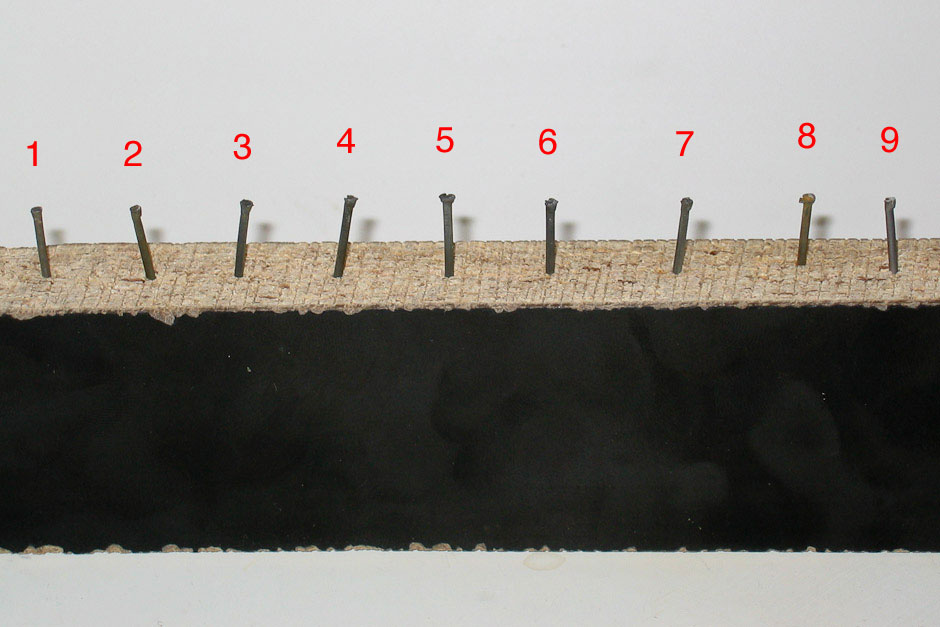
Now that we know what happens to the outside appearance of the nail, we now need to test its structural strength. The only test I could think of that I could do with my limited resources was to try to hammer each nail into some wood.
The results of the hammer and nail test were the same for each nail. Each nail had no problem being driven into the wood. Each nail took about four hits to be driven halfway into the wood (not including the four times I hit my thumb…the things I do for science!).
Conclusion
As I suspected, no nail of any kind was dissolved by any of the sodas.
At least that entry in the e-mail is false. Perhaps I should tackle some of the other entries…
Bibliography
The Coca-Cola Company
http://www.coca-cola.com/
Science Is Fun in the Lab of Shakhashiri - Chemical of the Week - Phosphoric Acid
http://scifun.chem.wisc.edu/chemweek/H3PO4/H3PO4.html
Gondar Design Science - Acids and alkalis—the pH scale
http://purchon.com/chemistry/ph.htm